38 cautionary and advisory labels for medicines
Labels on medicines and poisons - Department of Health Some medicines also require additional label warnings. For example, oral retinoids must have warnings about becoming pregnant. Sedation warnings. Medicines listed in Appendix K of the SUSMP (exernal site) must be labelled with a sedation warning when supplied to patients. Pharmacy cautionary advisory label 1 or label 1A should be used. Consumer Updates | FDA - U.S. Food and Drug Administration 28.07.2022 · The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Medicinal forms | Macrogol 3350 with potassium chloride, sodium ... There can be variation in the licensing of different medicines containing the same drug. Oral solution . All products. Show Cautionary and advisory labels. Label 13 . Dissolve or mix with water before taking. Gadewch i doddi mewn dŵr cyn ei gymryd. Electrolytes. May contain bicarbonate, chloride, potassium, sodium. Show Sugar free Movicol Liquid Forum Health Products Ltd Show …

Cautionary and advisory labels for medicines
Medicinal forms | Quetiapine | Drugs | BNF | NICE There can be variation in the licensing of different medicines containing the same drug. Forms available from special-order manufacturers include: oral suspension, oral solution, powder. Tablet. All products. Show Cautionary and advisory labels. Label 2 . Warning: This medicine may make you sleepy. If this happens, do not drive or use tools or machines. Do not drink alcohol . … FDA Drug Safety Communication FDA Briefing Information for the February 10-11, 2014 Joint Meeting of the Arthritis Advisory Committee and Drug Safety and Risk Management Advisory Committee. Available from:. Accessed December ... Medicinal forms | Mesalazine | Drugs | BNF | NICE There can be variation in the licensing of different medicines containing the same drug. Modified-release tablet. All products. Show Cautionary and advisory labels. Label 21 (does not apply to Pentasa® tablets) Take with or just after food, or a meal. Cymerwch gyda neu ar ôl bwyd. Label 25 (does not apply to Pentasa® tablets) Swallow this ...
Cautionary and advisory labels for medicines. British National Formulary - Wikipedia The British National Formulary (BNF) is a United Kingdom (UK) pharmaceutical reference book that contains a wide spectrum of information and advice on prescribing and pharmacology, along with specific facts and details about many medicines available on the UK National Health Service (NHS). Information within the BNF includes indication(s), contraindications, side effects, doses, … Outcome of the consultation on the proposed warning and advisory ... The Label Statements Database will be updated on 1 September 2022. Medicines released for supply in New Zealand after 1 March 2024 must have updated package labels that include the new warning and advisory statement. However, we encourage sponsors to update their labels before this date, if feasible. Medicinal forms | Oxycodone hydrochloride | Drugs | BNF | NICE There can be variation in the licensing of different medicines containing the same drug. Forms available from special-order manufacturers include: oral solution, solution for infusion. Tablet. All products. Show Cautionary and advisory labels. Label 2 . Warning: This medicine may make you sleepy. If this happens, do not drive or use tools or machines. Do not drink alcohol . Rhybudd: … BNFC (British National Formulary for Children) | NICE 27.07.2022 · Medicines approved by the NHS for Nurse Practitioner prescribing. Dental practitioners formulary . Medicines approved by the NHS for dental prescribing. Approximate conversions and units. Conversions and units tables. Includes growth chart with average weight and height, by gender and age (neonate, child and adult). Cautionary and advisory labels. …
Boxed warning - Wikipedia In the United States, a boxed warning (sometimes "black box warning", colloquially) is a type of warning that appears on the package insert for certain prescription drugs, so called because the U.S. Food and Drug Administration specifies that it is formatted with a 'box' or border around the text. The FDA can require a pharmaceutical company to place a boxed warning on the labeling … Medicinal forms | Mesalazine | Drugs | BNF | NICE There can be variation in the licensing of different medicines containing the same drug. Modified-release tablet. All products. Show Cautionary and advisory labels. Label 21 (does not apply to Pentasa® tablets) Take with or just after food, or a meal. Cymerwch gyda neu ar ôl bwyd. Label 25 (does not apply to Pentasa® tablets) Swallow this ... FDA Drug Safety Communication FDA Briefing Information for the February 10-11, 2014 Joint Meeting of the Arthritis Advisory Committee and Drug Safety and Risk Management Advisory Committee. Available from:. Accessed December ... Medicinal forms | Quetiapine | Drugs | BNF | NICE There can be variation in the licensing of different medicines containing the same drug. Forms available from special-order manufacturers include: oral suspension, oral solution, powder. Tablet. All products. Show Cautionary and advisory labels. Label 2 . Warning: This medicine may make you sleepy. If this happens, do not drive or use tools or machines. Do not drink alcohol . …


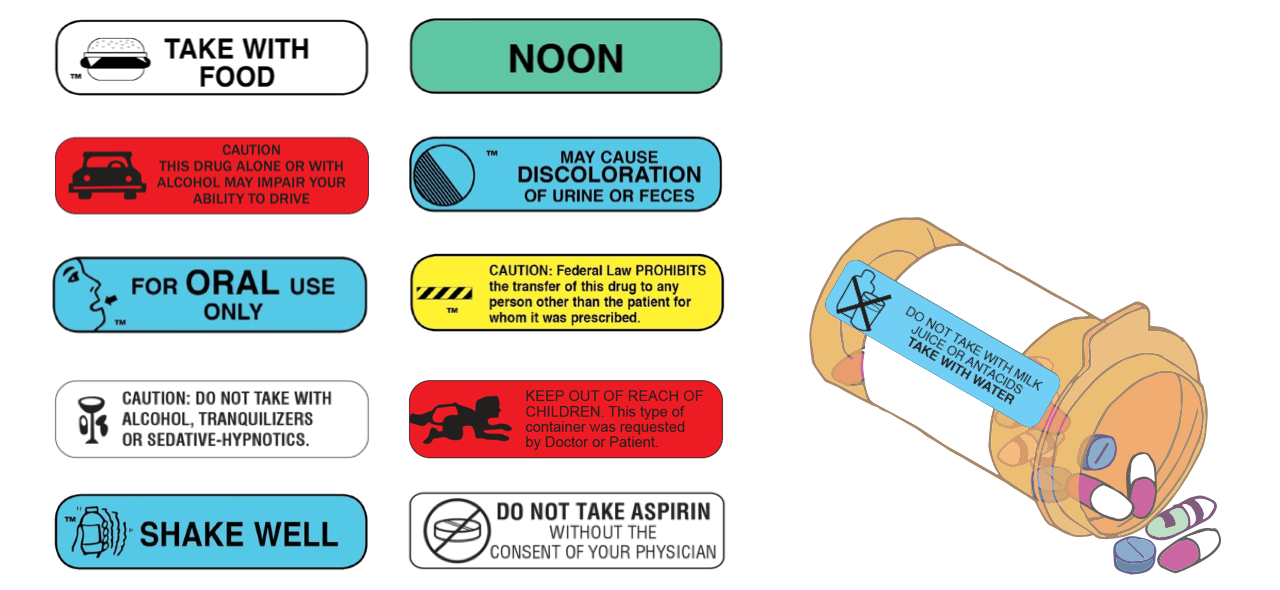







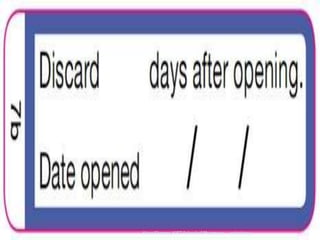



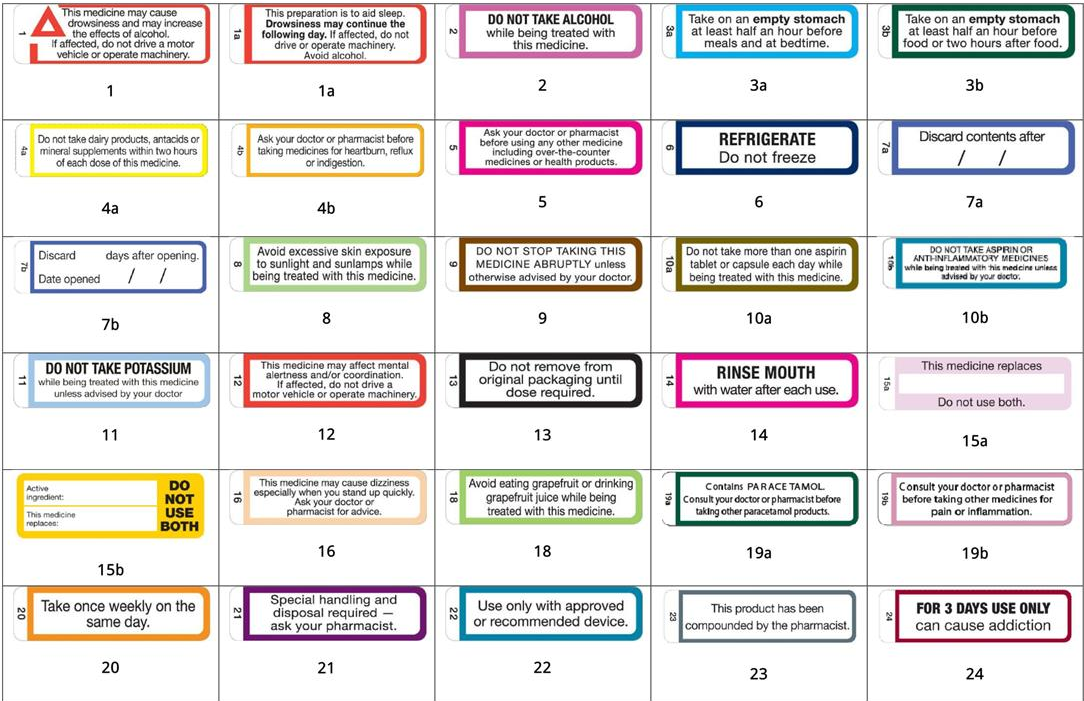











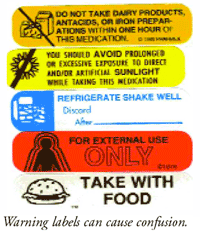

![PDF] Improving prescription drug warnings to promote patient ...](https://d3i71xaburhd42.cloudfront.net/e087ba4ebaa8ed043bf22bd2ab63292d6a05b9d4/2-Figure1-1.png)

Post a Comment for "38 cautionary and advisory labels for medicines"